Nearly half the world’s population is at risk of Malaria, and the Plasmodium parasite is becoming drug-resistant. Human IgGs are protective, but Plasmodium is complex, making it difficult to identify antigenic vaccine targets.
The limitations of ELISA
Researchers at KEMRI Wellcome Trust had expressed 117 P. falciparum antigens and intended to screen the panel in triplicate against 700 patient serum samples. ELISA is labour-intensive and time-consuming: profiling 700 serum samples would have taken the team more than eight months.
Faster screening
The principle behind protein arrays is similar to ELISA, and Arrayjet are experienced in transferring ELISA projects to microarray. To achieve this, Arrayjet Advance™ formulated a custom printing buffer, then determined the optimum sample dilution and deposition volume in a proof-of-concept study. The preliminary data were validated by KEMRI, and Arrayjet scientists designed an array to fit all 117 antigens in triplicate on each pad of a 24-pad slide (Arrayjet, AJC033; Figure 1). A 24-well slide module (Figure 2) could be superimposed to create a structure that resembles an ELISA plate.
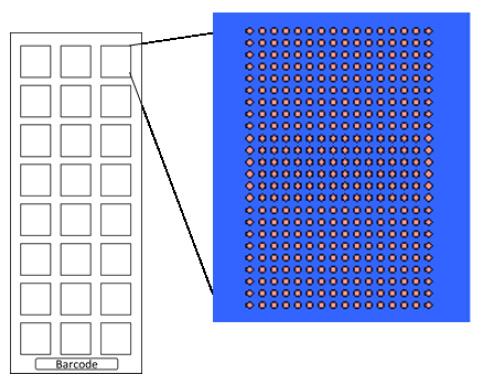

The original method would have required ~850 ELISA plates, but all 700 human serum samples and controls could be profiled on just 30 slides. By transferring to microarray, the entire screening project could be completed in a single day, instead of eight months with ELISA. Significant cost savings were made on test reagents and consumables.
Bigger batches: better reproducibility
Subsequently, the KEMRI team used this method to profile over 20,000 human serum samples by microarray, making it the largest malaria study of its kind. With such a large cohort, assay variation must be minimal and Arrayjet solved this issue in a number of ways:
- Outsourced printing made possible via Arrayjet Advance™ experts
- Instruments and components QC’d to CV < 5%
- Constant environmental control
- 1000 slides printed over five days, in a single batch
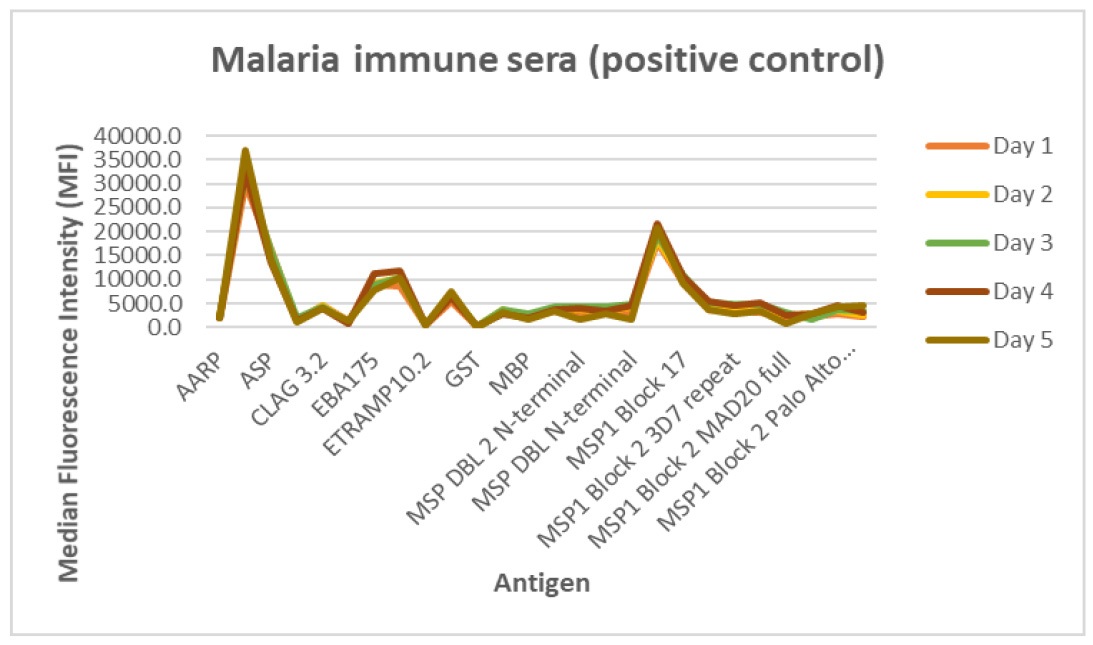
Antibody responses to control sera were measured to assess the reproducibility of five protein arrays printed on five consecutive days, under identical printing conditions, and using the same batch of recombinant proteins. Data in Figure 3 represent the median MFI values of triplicate readings obtained with malaria immune sera (MIS) to a subset of the antigens (n = 13).
Testimonial
“The combination of an excellent product in their printers and a responsive technical support team to assist with custom solutions for our project were two of the attributes that attracted us to work with Arrayjet. At every step of the design of a microarray chip, we had at our disposal a dedicated team of Application Specialists who were very cordial and responded to our many queries. It was on the back of this excellent support that we eventually purchased one of their microarray printers and we have enjoyed excellent technical training on the instrument and engineering support.”
Dr. James Tuju, Principal Investigator, KEMRI Wellcome Trust, Kenya


